NT Glass
B.A., Eckerd College, St. Petersburg, FL, 2013Abstract. The Rome ECO Center's garden furthers the center's environmental education initiative through the exhibition on native plant species. Knowledge of the soil nutrient composition of the garden will enhance future planting endeavors. The garden was divided into 70 plots based on a 10X10 foot grid, and each plot was tested for soil nutrients and pH level using a LaMotte Complete Soil Testing Kit. Test results found that the garden is low in Nitrogen but contains medium amounts of potassium and phosphorus. The average soil pH level is 6.85. Phosphorus is low at the garden's base but increases as one moves upwards away from the Center. High nitrogen content is rare and exists only in a few isolated pockets. High levels of nutrient content exist along the borders of the garden. For future gardening efforts I suggest the application of an N-fertilizer. It should also be noted that the cultivation of plants along the garden's borders is likely to be successful based on soil nutrient content in these areas.
1 Introduction
The ECO Center garden focuses on growth and exhibition of native plants for environmental education purposes. The garden includes various themes such as "Meadow" and "Rain Forest" sections and a shade garden. As of yet, no soil map exists of the ECO Garden.
The success of plant growth depends upon several factors. Of these factors a balanced soil pH level and ample soil content of nitrogen, phosphorus, and potassium are vital. Nitrogen is involved in almost all biochemical processes within plants. It is a component of chlorophyll and facilitates the formation of amino and nucleic acids and enzymes. Nitrogen also stimulates the plant to absorb and utilize other nutrients such as phosphorus and potassium. Phosphorus encourages root development and speeds the maturing process. It also increases the total biomass of the plant by stimulating rapid cell development, and thus also serves to increase the plant's resistance to disease. Finally, potassium contributes to the activation of various enzymes, the production of amino acids, the prevention of wilting, and plant resistance to disease. Soil pH has been included in this study as it is an excellent indicator of soil production potential. A soil map of pH levels and N-K-P content is thus beneficial to future gardening efforts and therefore to the environmental education initiative of Rome ECO Center.
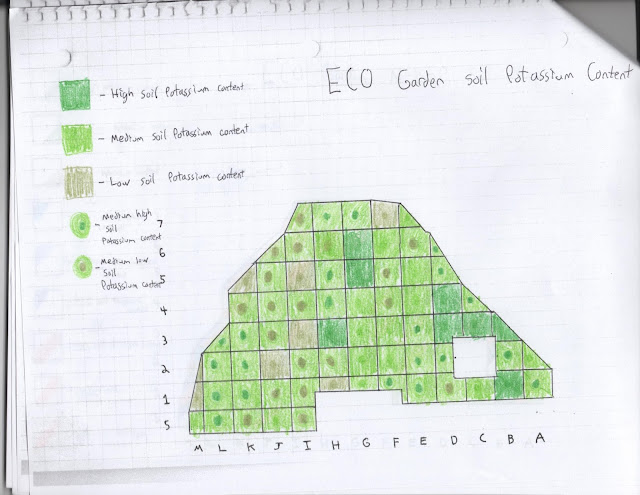
In this study I have divided the ECO Garden into 70 plots based on a 10X10 foot grid (Figure 1). Note that due to the triangular shape of the garden not all sections are equal in length of width. Each plot was tested for soil pH, nitrogen content, phosphorus content, and potassium content. The results have been used to create a soil nutrient and pH map (Figure 6).
2 Methods
I divided the ECO Garden into 70 plots based on a 10X10 foot grid overlaid across the garden's area
using measuring tape and flags. Measurements began in the west corner of the garden and moved in an eastward direction. The resulting map includes 13 columns at its widest and 8 rows at its longest. The columns are labeled alphabetically from right to left (column A to column M). The rows are labeled from bottom to top, beginning with row S (S represents "Shade Garden": the section of the garden directly adjacent to the ECO Center's main entrance) and continue upward to row 7. I obtained a soil sample from the center of each plot at a depth of 3 inches. Each soil sample was dried overnight on printing paper, removed of foreign objects, filtered, and tested for pH level and N-K-P content using a LaMotte Complete Soil Testing Kit.
3 Results
The following are the results of the soil testing, beginning at plot A1 (far west corner) and continuing right-to-left to plot I7 (far east corner).
Please note: plot C2 is encompassed by concrete.
A1: pH 7, Phosphorus low, Nitrogen low, Potassium medium high
B1: pH 7.5, Phosphorus low, Nitrogen trace, Potassium high
C1: pH 8, Phosphorus trace, Nitrogen trace, Potassium medium
D1: pH 7.75, Phosphorus low, Nitrogen trace, Potassium medium low
E1: pH 7.75, Phosphorus low, Nitrogen trace, Potassium medium high
F1: pH 7, Phosphorus trace, Nitrogen trace, Potassium medium
G1: pH 7.7.5, Phosphorus trace, Nitrogen trace, Potassium medium
H1: pH 7, Phosphorus trace, Nitrogen trace, Potassium low
I1: pH 6, Phosphorus trace, Nitrogen trace, Potassium medium low
I-S: pH 5.5, Phosphorus trace, Nitrogen trace, Potassium medium low
J1: pH 6.5, Phosphorus trace, Nitrogen trace, Potassium medium low
J-S: pH 6, Phosphorus medium, Nitrogen low, Potassium medium
K1: pH 6.5, Phosphorus trace, Nitrogen trace, Potassium medium high
K-S: pH 7, Phosphorus medium, Nitrogen trace, Potassium medium low
L1: pH 6.75, Phosphorus low, Nitrogen trace, Potassium medium high
L-S: pH 7, Phosphorus low, Nitrogen trace, Potassium Medium high
M1: pH 7, Phosphorus low, Nitrogen medium, Potassium low
M-S: pH 7, Phosphorus low, Nitrogen high, Potassium medium
A2: pH 6.5, Phosphorus low, Nitrogen high, Potassium medium
B2: pH 7, Phosphorus low, Nitrogen trace, Potassium medium high
D2: pH 7, Phosphorus low, Nitrogen trace, Potassium medium high
E2: pH 7, Phosphorus medium, Nitrogen trace, Potassium medium high
F2: pH 7, Phosphorus low, Nitrogen trace, Potassium medium
G2: pH 6.5, Phosphorus medium, Nitrogen trace, Potassium medium
H2: pH 7, Phosphorus trace, Nitrogen trace, Potassium medium
I2: pH 7, Phosphorus low, Nitrogen trace, Potassium medium
J2: pH 7, Phosphorus trace, Nitrogen trace, Potassium low
K2: pH 7, Phosphorus trace, Nitrogen trace, Potassium medium high
L2: pH 6.75, Phosphorus high, Nitrogen trace, Potassium medium high
M2: pH 6.5, Phosphorus medium, Nitrogen low, Potassium low
B3: pH 7, Phosphorus low, Nitrogen medium, Potassium high
C3: pH 7, Phosphorus low, Nitrogen low, Potassium high
D3: pH 7.5, Phosphorus low, Nitrogen trace, Potassium high
E3: pH 7.25, Phosphorus medium, Nitrogen low, Potassium medium high
F3: pH 7.25, Phosphorus low, Nitrogen low, Potassium medium
G3: pH 7, Phosphorus medium, Nitrogen trace, Potassium medium
H3: pH 7, Phosphorus low, Nitrogen trace, Potassium high
I3: pH 7.5, Phosphorus low, Nitrogen low, Potassium low
J3: pH 7, Phosphorus low, Nitrogen trace, Potassium medium low
K3: pH 7.5, Phosphorus trace, Nitrogen trace, Potassium medium high
L3: pH 7, Phosphorus medium, Nitrogen trace, Potassium medium high
C4: pH 6, Phosphorus low, Nitrogen medium, Potassium medium high
D4: pH 7.5, Phosphorus low, Nitrogen trace, Potassium high
E4: pH 7, Phosphorus low, Nitrogen trace, Potassium medium
F4: pH 6.5, Phosphorus medium, Nitrogen low, Potassium medium low
G4: oH 7, Phosphorus medium, Nitrogen trace, Potassium medium
H4: pH 7, Phosphorus medium, Nitrogen trace, Potassium medium high
I4: pH 6.5, Phosphorus low, Nitrogen trace, Potassium medium low
J4: pH 7.75, Phosphorus low, Nitrogen trace, Potassium medium low
K4: pH 7, Phosphorus trace, Nitrogen low, Potassium medium low
D5: pH 6, Phosphorus low, Nitrogen trace, Potassium medium high
E5: pH 6, Phosphorus low, Nitrogen trace, Potassium medium
F5: pH 6.5, Phosphorus low, Nitrogen trace, Potassium medium low
G5: pH 6.5, Phosphorus low, Nitrogen trace, Potassium high
H5: pH 7, Phosphorus medium, Nitrogen trace, Potassium medium
I5: pH 6.5, Phosphorus medium, Nitrogen low, Potassium low
J5: pH 7, Phosphorus low, Nitrogen trace, Potassium medium low
K5: pH 7, Phosphorus low, Nitrogen medium, Potassium low
D6: pH 7, Phosphorus low, Nitrogen low, Potassium medium high
E6: pH 6.75, Phosphorus low, Nitrogen trace, Potassium medium low
F6: pH 7, Phosphorus trace, Nitrogen trace, Potassium medium
G6: pH 6.5, Phosphorus trace, Nitrogen low, Potassium high
H6: pH 7.5, Phosphorus low, Nitrogen medium, Potassium high
I6: pH 7.5, Phosphorus low, Nitrogen high, Potassium medium low
J6: pH 8, Phosphorus trace, Nitrogen medium, Potassium medium low
E7: pH 7, Phosphorus low, Nitrogen low, Potassium medium
F7: pH 6.5, Phosphorus trace, Nitrogen trace, Potassium low
G7: pH 7.5, Phosphorus trace, Nitrogen trace, Potassium medium high
H7: pH 7.5, Phosphorus low, Nitrogen trace, Potassium medium high
I7: pH 7, Phosphorus low, Nitrogen low, Potassium medium low
The average pH level for the ECO Garden is 6.85. The average soil phosphorus content is medium low (50 lbs/acre), although more than half of the plots contain a low level of phosphorus. The average soil nitrogen content is low (15 lbs/acre). The average soil potassium content is medium (160 lbs/acre).
4 Discussion
Overall, the ECO Garden is low on nitrogen. Phosphorus and potassium content is medium. Row 1 has the least amount of phosphorus and contains only trace amounts of nitrogen. Phosphorus content is more prevalent in the center of the garden. Interestingly, plots that border the garden hold pockets of high nutrient content, possibly due to a lack of plant absorption. Pockets of high nitrogen content exist in plots M1, K5, J6, H6, I6, C4, B3, and A2. Phosphorus and nitrogen content are low in plots containing the dry creek bed, although potassium content appears unaffected. This may be explained by potassium's high natural occurrence rate in soils, which also explains the garden's high potassium content overall.
The rich presence of nutrients along the border of the garden presents an opportunity for the cultivation of border-plants in these areas. Such plants are likely to grow well and thrive in these plots provided adequate space is secured for ample root growth. The low presence of nitrogen throughout the garden may be remedied by adding appropriate amounts of nitrogen-rich fertilizer to the entire garden, although side effects may include increased weed growth.
Acknowledgements
I would like to thank Ben and Jason of the Rome ECO Center for encouraging this study and for providing the flags used to outline garden plots.
Figures
Figure 1: Grid outline of ECO Garden
Figure 2: pH content of ECO Garden
Figure 3: Phosphorus content of ECO Garden
Figure 4: Nitrogen content of ECO Garden
Figure 5: Potassium content of ECO Garden
Figure 6: N-K-P map of ECO Garden